

The primary endpoint was disease-free survival in the intention-to-treat population. Randomisation was stratified by previous neoadjuvant chemotherapy use, number of lymph nodes resected, pathological nodal status, tumour stage, and PD-L1 expression on tumour-infiltrating immune cells.

Patients were randomly assigned (1:1) using a permuted block (block size of four) method and interactive voice-web response system to receive 1200 mg atezolizumab given intravenously every 3 weeks for 16 cycles or up to 1 year, whichever occurred first, or to observation. No post-surgical radiotherapy or previous adjuvant chemotherapy was allowed. Patients not treated with neoadjuvant chemotherapy must have been ineligible for or declined cisplatin-based adjuvant chemotherapy. Patients had ypT2–4a or ypN+ tumours following neoadjuvant chemotherapy or pT3–4a or pN+ tumours if no neoadjuvant chemotherapy was received.
#Checkmate 274 trial#
In the IMvigor010 study, a multicentre, open-label, randomised, phase 3 trial done in 192 hospitals, academic centres, and community oncology practices across 24 countries or regions, patients aged 18 years and older with histologically confirmed muscle-invasive urothelial carcinoma and an Eastern Cooperative Oncology Group performance status of 0, 1, or 2 were enrolled within 14 weeks after radical cystectomy or nephroureterectomy with lymph node dissection. We aimed to evaluate atezolizumab as adjuvant therapy in patients with high-risk muscle-invasive urothelial carcinoma. Placebo in Muscle-Invasive Urothelial Carcinomaĭespite standard curative-intent treatment with neoadjuvant cisplatin-based chemotherapy, followed by radical surgery in eligible patients, muscle-invasive urothelial carcinoma has a high recurrence rate and no level 1 evidence for adjuvant therapy. (Funded by Bristol Myers Squibb and Ono Pharmaceutical CheckMate 274 number, NCT02632409.) In this trial involving patients with high-risk muscle-invasive urothelial carcinoma who had undergone radical surgery, disease-free survival was longer with adjuvant nivolumab than with placebo in the intention-to-treat population and among patients with a PD-L1 expression level of 1% or more. Two treatment-related deaths due to pneumonitis were noted in the nivolumab group. Treatment-related adverse events of grade 3 or higher occurred in 17.9% of the nivolumab group and 7.2% of the placebo group. Among patients with a PD-L1 expression level of 1% or more, the percentage of patients was 75.3% and 56.7%, respectively (hazard ratio, 0.55 95% CI, 0.39 to 0.79).
#Checkmate 274 free#
The percentage of patients who were alive and free from recurrence outside the urothelial tract at 6 months was 77.0% with nivolumab and 62.7% with placebo (hazard ratio for recurrence outside the urothelial tract or death, 0.72 95% CI, 0.59 to 0.89). The median survival free from recurrence outside the urothelial tract in the intention-to-treat population was 22.9 months (95% CI, 19.2 to 33.4) with nivolumab and 13.7 months (95% CI, 8.4 to 20.3) with placebo. Among patients with a PD-L1 expression level of 1% or more, the percentage of patients was 74.5% and 55.7%, respectively (hazard ratio, 0.55 98.72% CI, 0.35 to 0.85 P<0.001). The percentage of patients who were alive and disease-free at 6 months was 74.9% with nivolumab and 60.3% with placebo (hazard ratio for disease recurrence or death, 0.70 98.22% CI, 0.55 to 0.90 P<0.001). The median disease-free survival in the intention-to-treat population was 20.8 months (95% confidence interval, 16.5 to 27.6) with nivolumab and 10.8 months (95% CI, 8.3 to 13.9) with placebo.
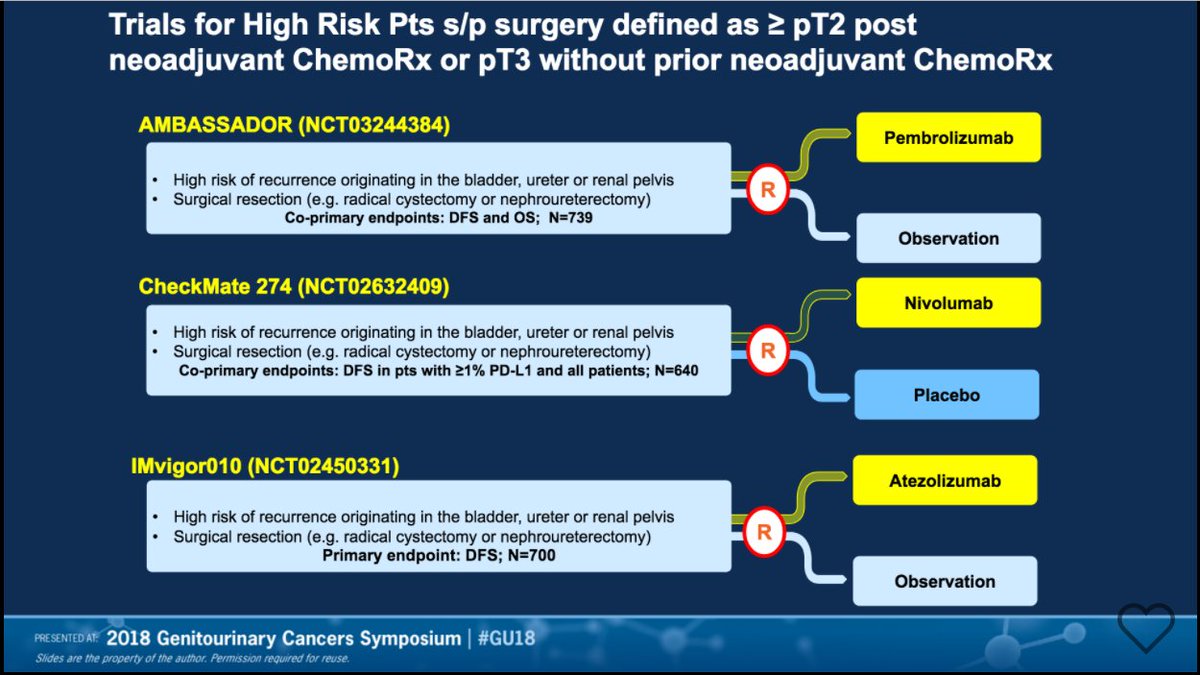

Survival free from recurrence outside the urothelial tract was a secondary end point.Ī total of 353 patients were assigned to receive nivolumab and 356 to receive placebo. The primary end points were disease-free survival among all the patients (intention-to-treat population) and among patients with a tumor programmed death ligand 1 (PD-L1) expression level of 1% or more. Neoadjuvant cisplatin-based chemotherapy before trial entry was allowed. In a phase 3, multicenter, double-blind, randomized, controlled trial, we assigned patients with muscle-invasive urothelial carcinoma who had undergone radical surgery to receive, in a 1:1 ratio, either nivolumab (240 mg intravenously) or placebo every 2 weeks for up to 1 year. The role of adjuvant treatment in high-risk muscle-invasive urothelial carcinoma after radical surgery is not clear.


 0 kommentar(er)
0 kommentar(er)
